


Next: Acknowledgements
Up: Structure Check Tutorial
Previous: Chirality in proteins and
Contents
Subsections
cis peptide bonds in proteins
In naturally occurring proteins most peptide bonds are in the trans
configuration (see Fig. 6). However, sometimes cis
peptide bonds do occur. The vast majority of cis peptides is observed at
a proline, Xaa-Pro, Xaa being any amino acid. But non-proline Xaa-non
Pro cis bonds are also found in proteins, although they occur much less
frequently than Xaa-Pro (see A. Jabs et al., JMB, 286, 291-304 (1999)).
Figure 6:
Structurally optimized 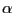 -helix containing a cispeptide
bond. The cis peptide bond is shown in CPK, while hydrogen bonds within the helix are
shown as thick dashed blue lines. The cis configuration of the peptide bond disrupts
the hydrogen bond network stabilizing the helix. Note, the hydrogen bond network is
broken not only locally.
-helix containing a cispeptide
bond. The cis peptide bond is shown in CPK, while hydrogen bonds within the helix are
shown as thick dashed blue lines. The cis configuration of the peptide bond disrupts
the hydrogen bond network stabilizing the helix. Note, the hydrogen bond network is
broken not only locally.
![\begin{figure}\begin{center}
\par
\par
\latex{
\includegraphics[scale=0.4]{FIGS/peptide}
}
\end{center}
\end{figure}](img13.gif) |
The configuration of the peptide bond is central to the sort of secondary structure the
protein backbone can adopt. It is easy to understand by imagining a normal 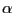 helix
and converting a trans peptide bond into its cis form. The result is that the hydrogen
bond network stabilising the helix is broken (see Fig. 6) and the helix will be unstable in long
simulations.
helix
and converting a trans peptide bond into its cis form. The result is that the hydrogen
bond network stabilising the helix is broken (see Fig. 6) and the helix will be unstable in long
simulations.
To demonstrate the usage of the plugin we will start by checking a structure consisting of
the protein EF-Tu in complex with a tRNA-Phe, although it is clear that in this example
only the protein part will contain peptide bonds. The used structure is based on the the
PDB structure 1OB2 in which errors have been introduced manually.
Currently, the cispeptide plugin can be used from the text console and through a
GUI. The available commands provided by the pugin can be obtained by typing Type cispeptide.
The following information should be printed in the console:
Usage: cispeptide <command> [args...]
Commands:
check -- identify cis peptide bonds
list -- list identified cis peptide bonds
minimize -- fix cis peptide bonds using energy minimization
move -- move specified atom to convert to trans
reset -- reinitialize plugin state
restrain -- generate NAMD extrabonds file to restrain peptide bonds
set -- define how to modify a given cis peptide bond
show -- visualize identified cis peptide bonds
In the same way the usage information for any of the provided commands
can be obtained. For example, typing cispeptide restrain will
show the syntax of the command that generates restraints for the
dihedral angle at the peptide bond which can be used in NAMD.
In the following, we will describe how to use the cispeptide
plugin through its GUI, but all of the tasks can also be accomplished
from the console.
- 1
- Load the files pept_testcase.psf and
pept_testcase.pdb into a new session of VMD.
- 2
- Open the cispeptide GUI by selecting Extensions
 Modeling
Modeling  Fix Cis Peptide Bonds in the
VMD Main menu. In the upper part of the GUI (see Fig. 7) the user can
specify the molecule and the atom selection to be tested.
Fix Cis Peptide Bonds in the
VMD Main menu. In the upper part of the GUI (see Fig. 7) the user can
specify the molecule and the atom selection to be tested.
Figure 7:
The cispeptide GUI window.
![\begin{figure}\begin{center}
\par
\par
\latex{
\includegraphics[scale=0.4]{FIGS/cispeptide_basic}
}
\end{center}
\end{figure}](img14.gif) |
- 3
- The button Check structure initializes the test. Peptides identified to be in
cis configuration are displayed in the Identified cis peptide bonds form (see
Fig. 8). In the present case there are 4 cis peptide bonds
identified in EF-Tu.
Figure 8:
Identified cis peptide bonds in the cispeptide GUI window.
![\begin{figure}\begin{center}
\par
\par
\latex{
\includegraphics[scale=0.4]{FIGS/cispeptide_errors}
}
\end{center}
\end{figure}](img15.gif) |
- 4
- By selecting an entry in the Identified cis peptide bonds form and hitting the
button Show selected cis peptide bond it is possible to visually inspect all the
individual identified cis peptides. In the created representation (see
Fig. 9) the cis peptide bond is highlighted in CPK representation.
Figure 9:
Representation of the cis peptide bond
between Asn 51 and Ala 52 within EF-Tu
![\begin{figure}\begin{center}
\par
\par
\latex{
\includegraphics[scale=0.4]{FIGS/cispeptide_show}
}
\end{center}
\end{figure}](img16.gif) |
WARNING: The cispeptide plugin identifies all cis peptide bonds.
As noted in the introduction, there are cases in which cis peptides do occur in
proteins. Thus, it should be checked if a given cis peptide is real or is a
mistake. This can be done by literature research, by comparing high resolution
crystallographic models, or by comparing crystallographic models of similar proteins.
- 1
- If the shown peptide bond is supposed to have a trans configuration,
the user can tag the atom to be moved to flip the configuration from cis to trans.
This is done by hitting the button hydrogen or oxygen.
WARNING: The choice which atom to move is crucial and has to be made based on the
local structure around the peptide under consideration. The decision is particularly
critical helices and 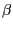 -sheets: the relaxed backbone should integrate in the
existing hydrogen bond network characteristic for the specific secondary structure motif
(see Fig. 6).
-sheets: the relaxed backbone should integrate in the
existing hydrogen bond network characteristic for the specific secondary structure motif
(see Fig. 6).
If the identified cis peptide bond is supposed to be there, nothing needs to be done.
It is also possible to untag a tagged atom by selecting the corresponding cis peptide
and hitting none.
- 2
- Once a cis peptide bond has been inspected, and the atom to be moved has
been tagged, the actual moving of the atom can be executed by hitting
the button Move tagged atoms for selected cis peptide bonds.
- 3
- Since the described procedure (simple moving of one atom)
generates an unphysical geometry of the molecule, it is necessary to
optimize the structure using an MD force field. The cispeptide
plugin uses the AutoIMD plugin for this purpose. This final step
is accomplished by selecting the cis peptides which should be
relaxed and hitting the button Minimize/equilibrate selected
cis peptide bonds. This will open the AutoIMD Controls window
(see Fig. 10).
Figure 10:
AutoIMD window called by cispeptide plugin.
![\begin{figure}\begin{center}
\par
\par
\latex{
\includegraphics[scale=0.4]{FIGS/autoimd_pept_basic}
}
\end{center}
\end{figure}](img18.gif) |
- 4
- Select Settings
 Minimization Mode in the
AutoIMD Controls window and hit then the Submit
button. Once the AutoIMD session started, hit the Connect button and a minimization will start. Finish the
simulation when the structure relaxed. Usually, it takes up to a few
thousand steps until atoms do not move anymore. Is the structure minimized, hit
the Finish in the AutoIMD Controls window.
For more details about using the AutoIMD plugin, the user is
referred to the AutoIMD user's guide
Minimization Mode in the
AutoIMD Controls window and hit then the Submit
button. Once the AutoIMD session started, hit the Connect button and a minimization will start. Finish the
simulation when the structure relaxed. Usually, it takes up to a few
thousand steps until atoms do not move anymore. Is the structure minimized, hit
the Finish in the AutoIMD Controls window.
For more details about using the AutoIMD plugin, the user is
referred to the AutoIMD user's guide
http://www.ks.uiuc.edu/Research/vmd/plugins/autoimd/.
- 5
- WARNING: Depending on the quality of the initial structure, it can happen that not
all errors can be fixed in one run. Thus, it is important to check the final result again
and correct the remaining errors, if necessary. In order to check the structure after the
performed minimization/equilibration using IMD, the cispeptide plugin should be
reset. This can be done by hitting the Reset cispeptide plugin button at the
bottom of the cispeptide window. Note, in the current implementation it will be
necessary to select the molecule again in the top part of the cispeptide window.
Note also that if the molecule to correct is solvated, it may be necessary to equilibrate the
minimized parts of the structure. This can be done by selecting Settings
 Equilibration Mode and submitting a new AutoIMD run.
Equilibration Mode and submitting a new AutoIMD run.
- 6
- Finally, it is a good advice to minimize the structure in the very last step.
This should be done by selecting Settings
 Minimization Mode in the
AutoIMD Controls window, which is still open from the last AutoIMD session,
and hit then the Submit button. As mentioned in the introduction, additional
restraints during the correction of cis peptides. The restraints will be used always
when AutoIMD is started from the cispeptide window. The equilibrium values
for these restraints are very approximate and bring the configuration in the correct
region. Thus, in order to obtain a optimized structure, it is necessary NOT to use the
restraints defined by cispeptide. This is achieved by reusing the AutoIMD
window from the previous minimization/equilibration; the restraints will not be taken into
account.
Minimization Mode in the
AutoIMD Controls window, which is still open from the last AutoIMD session,
and hit then the Submit button. As mentioned in the introduction, additional
restraints during the correction of cis peptides. The restraints will be used always
when AutoIMD is started from the cispeptide window. The equilibrium values
for these restraints are very approximate and bring the configuration in the correct
region. Thus, in order to obtain a optimized structure, it is necessary NOT to use the
restraints defined by cispeptide. This is achieved by reusing the AutoIMD
window from the previous minimization/equilibration; the restraints will not be taken into
account.
- 7
- Once all minimization/equilibration steps are performed, the corrected structure
should be saved. This can be done in the following way. Select the molecule in the VMD main window.
Create a representation containing all atoms in the Graphical Representations window.
Select File
 Save Coordinates in the VMD main window.
Select all in the Selected atoms and hit Save. Finally, specify the file
name in the Chose filename to save trajectory and hit OK.
Save Coordinates in the VMD main window.
Select all in the Selected atoms and hit Save. Finally, specify the file
name in the Chose filename to save trajectory and hit OK.
As mentioned in the introduction, structure optimizations and MD protocols applying
external forces can flip the configuration of a peptide bond in the the simulated
structure. To preserve peptide configuration, the cispeptide plugin offers the
possibility to generate restraints in form of extrabonds which can be used in a NAMD
simulations. This feature currently is available only from the console.
- 1
- Assuming the structure of interest is loaded in the top molecule in VMD and one wishes to
obtain restraints for all peptide bonds in the system, the command reads
cispeptide restrain -o peptide-extrabonds.txt
The file peptide-extrabonds.txt contains now one dihedral
restraint for each peptide bond. The equilibrium value of this dihedral is set to the
value present in the used structure.
WARNING: Note, the specified restraints modify the potential
energy function of the system, and thus should not be used in
equilibrium production runs. Rather, these restraints are supposed to
be used only in simulations in which large forces occur due to poor
initial geometry or due to externally applied forces, e.g., MDFF or
TMD. Note also that although the final structure resulting from such
simulations will be correct, the behavior of the system during the
simulation is artificial.



Next: Acknowledgements
Up: Structure Check Tutorial
Previous: Chirality in proteins and
Contents
school@ks.uiuc.edu
![\begin{figure}\begin{center}
\par
\par
\latex{
\includegraphics[scale=0.4]{FIGS/peptide}
}
\end{center}
\end{figure}](img13.gif)
![\begin{figure}\begin{center}
\par
\par
\latex{
\includegraphics[scale=0.4]{FIGS/peptide}
}
\end{center}
\end{figure}](img13.gif)
![]() helix
and converting a trans peptide bond into its cis form. The result is that the hydrogen
bond network stabilising the helix is broken (see Fig. 6) and the helix will be unstable in long
simulations.
helix
and converting a trans peptide bond into its cis form. The result is that the hydrogen
bond network stabilising the helix is broken (see Fig. 6) and the helix will be unstable in long
simulations.
![]() -sheets: the relaxed backbone should integrate in the
existing hydrogen bond network characteristic for the specific secondary structure motif
(see Fig. 6).
-sheets: the relaxed backbone should integrate in the
existing hydrogen bond network characteristic for the specific secondary structure motif
(see Fig. 6).