Click to view the full-size image
Image size:
1.6MB
Made with VMD
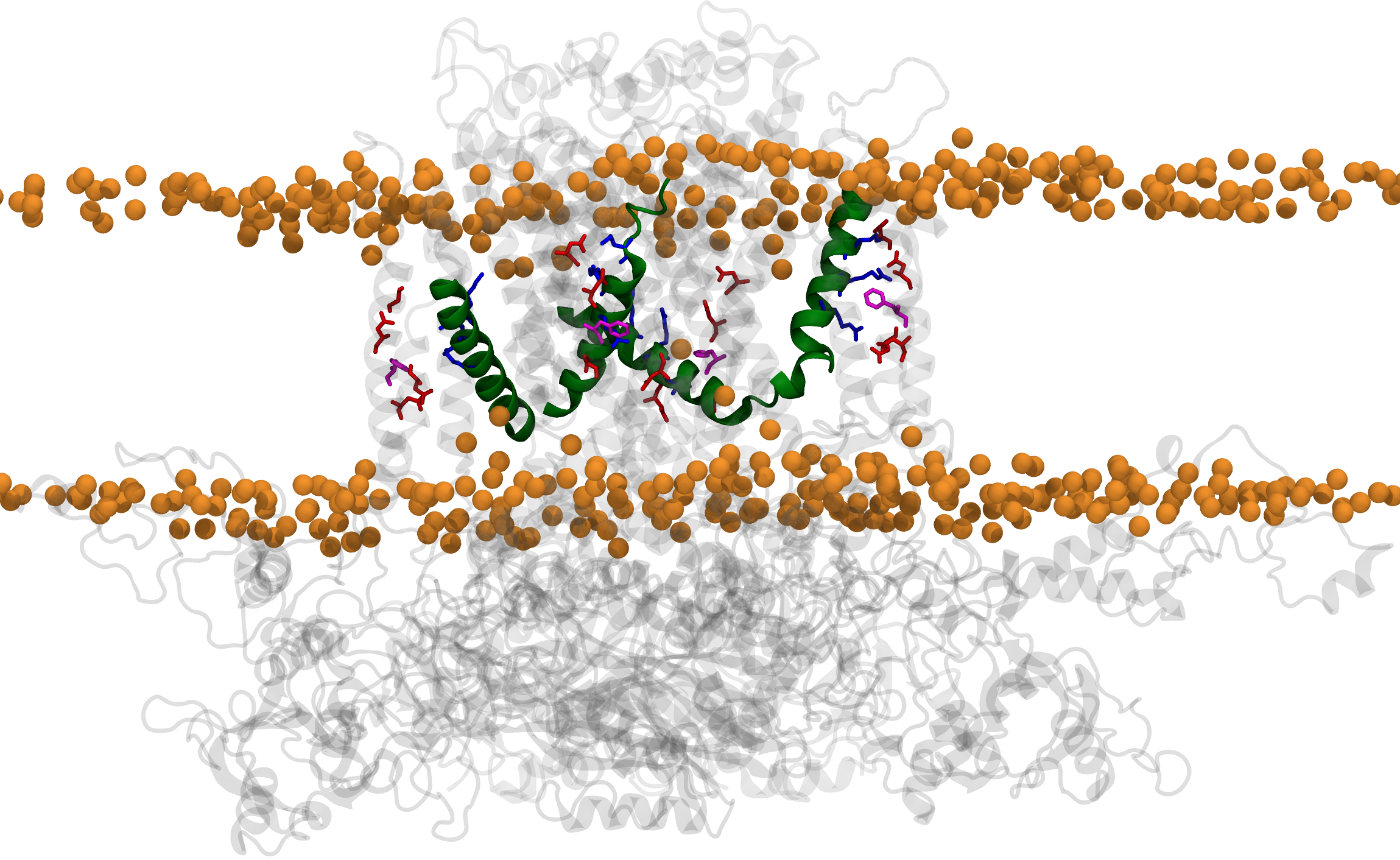
The human hERG potassium channel is essential for maintaining normal cardiac rhythm. Mutations of hERG, particularly in its voltage‑sensing arginines, can lead to long‑QT syndrome, a condition characterized by potentially fatal arrhythmias. However, the structural basis of how these mutations impair the voltage sensing of the channel has remained elusive. In our recent Nature Communications study, we combined imaging and single‑molecule experiments with molecular dynamics simulations to capture the intermediate conformations of the voltage‑sensing domains. Simulations were visualized and analyzed using VMD, while simulations were run in NAMD. Our results reveal that disease‑linked mutations alter charge transfer within the sensor, hindering movement and thereby disrupting channel gating. These insights help explain the molecular basis of arrhythmias and suggest strategies to restore hERG channel function.
The Future of Biomolecular Modeling
Magnetic Sense of Birds
Announcements
Seminars
Remembering Klaus Schulten
Recent Publications All Publications
- Drug-bound outward-facing conformation of a heterodimeric ABC exporter suggests a putative mechanism of drug translocation. Nat. Commun., 16:10403. 2025.
- Roles of the membrane-binding motif and the C-terminal domain of RNase E in localization and diffusion in E. coli. eLife, 14:RP105062. 2025.
- Ca2+ Stoichiometry Controls the Binding Mode of the PKCα C2 Domain to Anionic Membranes. J. Phys. Chem. B, 129(39):9893–9903. 2025.
- A to-do list for realizing the sequence-to-function paradigm of proteins. Curr. Opin. Struct. Biol., 93:103119. 2025.
- Voltage sensor conformations induced by LQTS-associated mutations in hERG potassium channels. Nat. Commun., 16:7126. 2025.
- Cryo-EM structure of the tissue factor/factor VIIa complex with a factor X mimetic reveals a novel allosteric mechanism. Blood, 2025.
- An Orchestrated Interaction Network at the Binding Site of Human SERT Enables the Serotonin Occlusion and Import. Biochemistry, 64(16):3652–3662. 2025.
Highly Cited
Click here for other highly cited papers