Bacterial Toxin Alpha-Hemolysin
Alpha-Hemolysin: Self-Assembling Transmembrane Pore
 Alpha-hemolysin, a self-assembling bacterial toxin.
Alpha-hemolysin, a self-assembling bacterial toxin.
In its fight for resources, bacterium
Staphylococcus aureus secretes alpha-hemolysin monomers that
bind to the outer membrane of susceptible cells. Upon binding, the
monomers oligomerize to form a water-filled transmembrane channel that
facilitates uncontrolled permeation of water, ions, and small organic
molecules. Rapid discharge of vital molecules, such as ATP,
dissipation of the membrane potential and ionic gradients, and
irreversible osmotic swelling leading to the cell wall rupture
(lysis), can cause death of the host cell. This pore-forming property
has been identified as a major mechanism by which protein toxins can
damage cells. The name alpha-hemolysin derives from early
observations that established lytic activity of the toxin on red blood
cells. It is expected now that, if applied in sufficient dosage,
alpha-hemolysin can permeate any mammalian cell membrane.
Although for most of the human population secretion of alpha-hemolysin
does not pose a serious health risk, severe staphylococcal infection
can cause hemostasis
disturbances, thrombocytopenia, and pulmonary
lesions (![]() ).
The crystallographic structure
(
).
The crystallographic structure
(![]() )
of the assembled
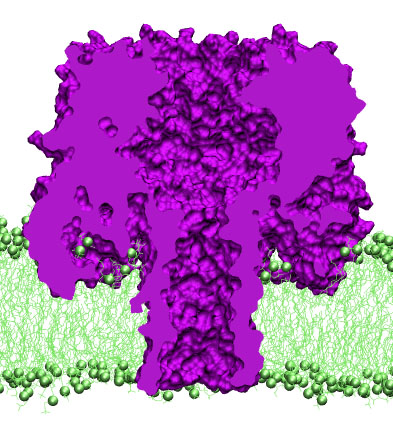
alpha-hemolysin revealed a heptametric organization of the channel.
The protein has a mushroom-like shape, with a 50A beta-barrel stem
protruding from the cap domain through the lipid bilayer into the
cell's interior. The cap of the protein conceals a large vestibule
connected to the cell's exterior through a large opening at the top of
the cap. The narrowest (1.4~nm in diameter) part of the channel is
located at the base of the stem, where the beta-barrel pore connects
to the vestibule. Seven side channels lead from the vestibule to the
cell's exterior, exiting near the membrane surface. The figure
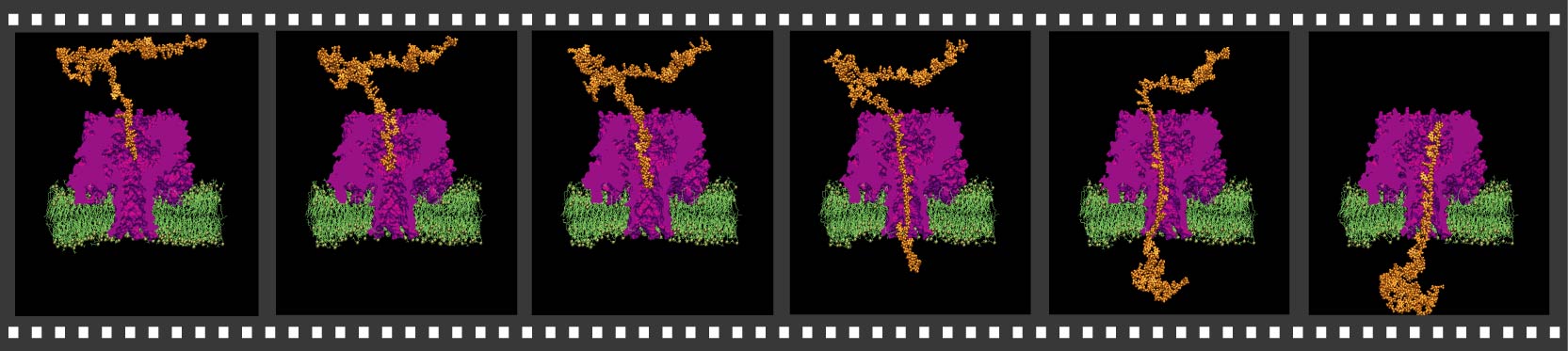
illustrates the 268,000-atom model of alpha-hemolysin in its native
environment - a lipid bilayer.
)
of the assembled
alpha-hemolysin revealed a heptametric organization of the channel.
The protein has a mushroom-like shape, with a 50A beta-barrel stem
protruding from the cap domain through the lipid bilayer into the
cell's interior. The cap of the protein conceals a large vestibule
connected to the cell's exterior through a large opening at the top of
the cap. The narrowest (1.4~nm in diameter) part of the channel is
located at the base of the stem, where the beta-barrel pore connects
to the vestibule. Seven side channels lead from the vestibule to the
cell's exterior, exiting near the membrane surface. The figure
illustrates the 268,000-atom model of alpha-hemolysin in its native
environment - a lipid bilayer.
- Click here for a movie (mpeg, [an error occurred while processing this directive]) showing alpha-hemolysin assembled with a lipid bilayer.
Biotechnological Applications of Alpha-Hemolysin
Several properties of alpha-hemolysin make this membrane channel suitable for various biotechnological applications: assembled alpha-hemolysin is stable over a wide range of pH and temperature, its transmembrane pore stays open at normal conditions, alpha-hemolysin can bind to various biological or synthetic lipid bilayers, the binding proceeds spontaneously and does not require specific ionic conditions.

Delivery Systems.
The transmembrane pore of alpha-hemolysin can facilitate controlled delivery of
ions and small organic compounds such as sugars or nucleotides across
a cell's plasma membrane or through the walls of synthetic lipid
vesicles. Using genetically engineered alpha-hemolysins, for which assembly and conductance can be
triggered or switched on or off by external biochemical or physical
stimuli including light, a lipid bilayer can be made permeable for
small solutes at will
(![]() ,
,
![]() ).
).
Stochastic Sensors.
Suspended in a lipid bilayer, an alpha-hemolysin channel becomes a stochastic
sensor when a molecular adapter is placed inside its genetically
re-engineered stem, influencing the transmembrane ionic current
induced by an applied voltage bias. Reversible binding of analytes to
the molecular adapter transiently reduces the ionic current. The
magnitude of the current reduction indicates the type of analyte,
while the frequency of the current reduction intervals reflects
analyte concentration. Such stochastic sensors were demonstrated to
simultaneously measure, with a single sensor element, concentrations
of several organic analytes
(![]() )
and solution concentrations
of two or more divalent metal ions
(
)
and solution concentrations
of two or more divalent metal ions
(![]() ).
The nanometer size pore of alpha-hemolysin was used in another type of stochastic sensor to simultaneously determine concentrations of two different
proteins
(
).
The nanometer size pore of alpha-hemolysin was used in another type of stochastic sensor to simultaneously determine concentrations of two different
proteins
(![]() ).
).
DNA sequencing. The
transmembrane pore of alpha-hemolysin can conduct not only
small solutes, but also rather big (tens of kDa) linear
macromolecules. Thus, driven by a transmembrane potential, DNA or RNA
strands can translocate through the pore of alpha-hemolysin, producing
the ionic current blockades that reflect the chemical structure of
individual strands
(![]() ). Statistical analysis of many such
blockage currents allowed the researchers to discriminate different
sequences of RNA
(
). Statistical analysis of many such
blockage currents allowed the researchers to discriminate different
sequences of RNA
(![]() ) and DNA
(
) and DNA
(![]() )
homopolymers, as well as the segments of purine and pyrimidine
nucleotides within a single RNA molecule
(
)
homopolymers, as well as the segments of purine and pyrimidine
nucleotides within a single RNA molecule
(![]() ).
A single nucleotide resolution has been demonstrated for DNA
hairpins
(
).
A single nucleotide resolution has been demonstrated for DNA
hairpins
(![]() ,
,
![]() ),
raising the prospect of creating a nanopore sensor capable of reading
the nucleotide sequence directly from a DNA or RNA strand.
),
raising the prospect of creating a nanopore sensor capable of reading
the nucleotide sequence directly from a DNA or RNA strand.
Imaging Alpha-Hemolysin with Molecular Dynamics
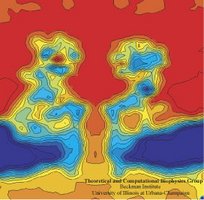
In a biological cell, membrane channels act like miniature valves regulating the flow of ions and other solutes between intracellular compartments and across the cell's boundary. Assembled in complex circuits, they generate, transmit, and amplify signals orchestrating cell function. To investigate how membrane channels work, life scientists, using an extremely fine pipette, isolate a tiny patch of a cell membrane and, in so-called patch clamp measurements, determine electric currents in response to applied electric potentials. Dramatic increase in computational power and its efficient utilization by NAMD allows one today to reproduce such studies computationally, calculating the permeability of a membrane channel to ions and water directly from its atomic structure. In what is one of the largest molecular dynamics simulation to date, described in a recent paper as well as on our web site (here), one copy of the membrane channel alpha-hemolysin, submerged in a lipid membrane and water, was subjected to an external electric field that drove ions and water through the channel. The calculations produced also an image of the electrostatic potential across the channel (see figure).
Publications
Investigators
Related TCB Group Projects
Page created and maintained by Aleksei Aksimentiev.