Actin
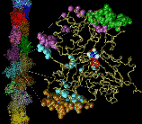
Fig 1: Click on the picture to get a larger, 113 kByte image of the inter-actin contact surfaces and the actin filament.
Actin filaments are dynamic polymers whose ATP-driven assembly in the cell cytoplasm drives shape changes, cell locomotion and chemotactic migration. Actin filaments also participate in muscle contraction.
Actin's "Back Door"
The mechanical properties of the filaments can be altered by actin-binding agents such as the toxin phalloidin from the mushroom "Amanita phalloides". Phalloidin binding to actin has been shown to delay the release of inorganic phosphate after ATP hydrolysis (Dancker & Hess, Biochim. Biophys. Acta, 1990, 1035:197). Conversely, no effect of inorganic phosphate on the phalloidin binding kinetics has been observed (De La Cruz & Pollard, Biochemistry, 1994, 33:14378). Our research goal was to resolve this seemingly paradoxical situation by identifying the dissociation pathway of the phosphate.
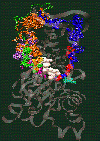
Fig. 2: Click on the picture to get a 109 kByte image of the diffusion of waters molecules into the nucleotide binding site of actin [visualized with the program VMD (side view)].
The simulation of monomeric actin required the development of a proper force field for ATP and ADP nucleotides (parameters available by email). The major discovery was the diffusion of water molecules into the buried nucleotide binding site along two distinct pathways (Fig. 2). Of special interest is the "back door" diffusion pathway which we believe to be relevant to the dissociation of the phosphate after hydrolysis. Our hypothesis for phosphate release would explain the seemingly paradoxical kinetics of actin-phalloidin interaction. Phalloidin, whose position in the F-actin filament structure is known, would in our model block the exit of the back door pathway and, thus, delay the release of the phosphate.
ATP and the Mg++ ion are represented as white spheres in the center of the protein, which is rendered as a transparent ribbon. The color of the traces of the water oxygens codes for different simulation trajectories. The Figure reveals two water diffusion pathways with the "back door" pathway on the right.
Actin Phosphate Release - The Movie
In order to substantiate our back door hypothesis of actin phosphate dissociation we studied further the dissociation of the nucleotide and the role of the proposed back door pathway for actin's enzymatic activity. To reveal the microscopic processes underlying the release of the inorganic phosphate, we explored possible release pathways in steered molecular dynamics simulations by measuring adhesion forces when pulling the substrate out of its binding pocket. The resulting structural prediction of the dissociation mechanism has important implications also for other putative back door enzymes such as the ATP-driven motor protein myosin (Yount et al., Biophys J., 1995, 68:44s).
Quicktime Movies
Total Length: 4 minutes
- 160x120 pixel resolution [ part 1 | part 2 | part 3 | part 4 | part 5 ] (22 MBytes total!)
- 320x240 pixel resolution [ part 1 | part 2 | part 3 | part 4 | part 5 ] (82 MBytes total!)



